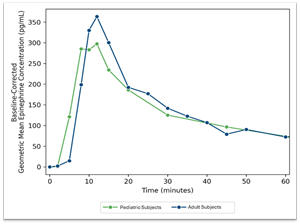
Aquestive Therapeutics Announces Positive Topline PK Results from its Pediatric Study and Completes the NDA Submission ...
Reports positive pharmacokinetic (PK) results from the recently completed pediatric study for Anaphylm™ Completes submission of its New Drug Application (NDA) for Anaphylm to the FDA; NDA acceptance expected in Q2 2025 WARREN, N.J., April 01, 2025 (GLOBE …
















